Sponges exhibit great plasticity due to their totipotent nature (Gaino et al. 1999). When dissociated, sponge cells are known to differentiate into specialised cell types and aggregate to reform the sponge body (Kamerling and Carvalho de Souza 2001). Archaeocytes are generally the cell that initiates this process. This is useful in asexual reproduction for budding and fragmentation. Upon dissociation, cells from different individuals would have the ability to recognise themselves and rejoin with the same cells from the same individual (Kamerling and Carvalho and de Souza 2011). As sponges live amongst many other organisms, in close proximity, self-non-self recognition becomes useful.
Methods
Sample collection
Coral rubble was collected on Heron Island Reef (North Beach and Fourth Point) during low tide. Coral rubble was broken using a hammer and chisel to determine whether sponge was present in the rock.
Image of Heron Island and sampling area North Beach
(Source:BIOL3211 handbook)
Cell aggregation
In order to dissociate the sponge cells, as much sponge tissue as possible was removed from the substrate and placed into a modified syringe. The tip of a regular 100ml syringe was cut off and a fine mesh was placed over the bottom by melting the mesh onto the syringe over a heating plate. Filtered seawater was then placed into the syringe with the sponge tissue. The cells were then squeezed into a 6 well plate also containing filtered seawater.
A twelve hour our time lapse was then set up using a dissecting microscope to determine if cell aggregation occurred.
Cell squeeze was repeated and centrifuged for ten minutes until a pellet was formed. Pellet was suspended in the supernatant using a pipette and 10microlitres was pipetted from the centrifuge to onto a microscope slide. Cell aggregation was recorded under a compound microscope with a five hour time lapse.
Self-non-self recognition
Two sponges from different individuals were removed from the substrate and a cell squeeze was performed as above with the exception of filtered seawater being replaced with calcium magnesium free seawater in both the syringe and the well plate. Calcium magnesium free seawater was used as itslows the process of cell aggregation, ensuring cells did not reaggregate during preparation.
The calcium magnesium free seawater containing the dissociated cells was centrifuged for ten minutes until a pellet was formed. Cells from both individuals were stained with either DAPI or CMFDA in order to differentiate the two individuals. Cells were then washed and resuspended in seawater and left to aggregate.
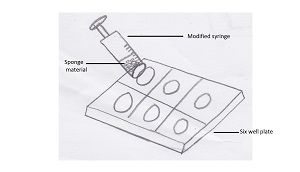 Image showing cell squeeze method
Image showing cell squeeze method
Results
Cell aggregation
Some aggregation of sponge cells were observed during the time lapse. Images are shown below. Filopodial extensions attracting neighbouring cells are usually seen in the process of cell aggregation, however these were not observed.
Self non self
As the dye did not adequately stain the sponge cells, self-non-self recognition of the sponge individuals was not observed. Therefore, it is not known whether this occurred in Cliona sp.
During the removal of sponge material from its attached substrate,it was noted that the sponge changed colour from yellow to dark purple. A purple dye-like substance was also produced from the sponge that stained fingers upon handling of the sponge during removal from the substrate and also stained the water during cell squeeze. This strange colour change doesn’t appear to occur in any other Clionaids. However, the secretion of a purple dye was noted in C. tinctoria, although this Clionaid is already purple in colour and the properties of the dye aren’t known(Schonberg 2000).
Other organisms that exhibit a similar purple colour change include the marine gastropods Bolinus brandaris and Dicathais orbota, where their toxic mucous secretion becomes purple in air due to a response to oxygen exposure. The chemical responsible for this reaction is known as Tyrian purple, a dye that is found in the hyperobranchial glands of these predatory marine gastropods (Benkendorff2013; Tanoue et al. 2001). In contrast, however, the body of this Cliona sp. only produced the colour change when exposed to some kind of aggravation such as scalpel prodding to remove tissue, or in the presence of ethanol, and no colour change was seen just to oxygen exposure. Also of note, after constant handling of Cliona sp. for a one week period with bare hands, skin irritation such as flaking occurred. Therefore, it is suggested that this purple dye may be caused as a result of a chemical defence response by the sponge and used as a mechanism to deter predators. Due to the nature of the sponge, this substance may also be used in its bioerosion process, as no chemical that is involved has yet to be identified. Unfortunately, no photo evidence of this reaction was taken.
Further research into the mechanism behind this reaction and the properties of this dye, should be conducted.